Back to the Basics.
Instead of complex things to do with physics, I thought i'd touch up on some chemistry, and try to help those revising for their January exam, like me for example. Reposting on this blog is purely for my own revision, but I thought i'd post it as my teacher says the best way to learn something is to go at it like you're teaching someone else it, so I thought I would actually try to teach others it.
The Basics.
Our old friend Mr. Atom - not Mr. Plum Pudding, Mr. Atom.
To start off, while doing chemistry - try not to mix Cu with CU - Cu is copper where as CU is carbon and uranium, and that's a pretty bad mistake to make.
An atom is composed primarily of 3 things - a proton, a neutron and an electron but you should all know that. A proton has a relative mass of 1, a neutron of basically 1 and an electron of 1/1800 - so its a lot smaller, it barely has any mass at all. Protons and neutrons are made up of quarks, I wont speak about them, but I/we did do a "lesson" so to speak on them a while back, here. Protons and neutrons are found in the nucleus/nucleon and electrons are found 'orbiting' the nucleus or in an orbital. A proton is a positive particle and a neutron [like the word neutral] is neutral - REMEMBER, IT IS NOT NEGATIVE. Although it may have a dipole moment but that is not important. An electron is a negative particle. Since most atoms have an equal number of protons and electrons, they are neutral altogether - unless they are an ion... but i'll go over that soon.
 Atoms are arranged neatly in order of groups and periods - a group represents how many atoms an element has on its outer shell and a period tells us how many shells it has. Group 0 is used because not all shells can contain 8, basically and this states that it has a full outer shell - in basic terms.
Atoms are arranged neatly in order of groups and periods - a group represents how many atoms an element has on its outer shell and a period tells us how many shells it has. Group 0 is used because not all shells can contain 8, basically and this states that it has a full outer shell - in basic terms.
Groups go down and periods go across. The first period contains helium, and it is in group 0. Group 0 means it has a full outer shell and is thus stable and does not need to gain any more electrons - and since it is in the first period, it only has one shell - and this is correct, helium has 2 electrons on its first energy level, and does not need any more electrons.
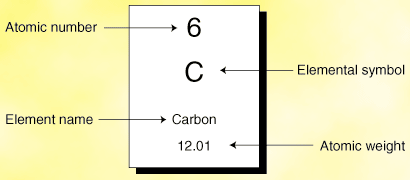
On the periodic table you generally have two numbers, one above the element and one below.
As shown by the diagram to the right, the number above is the "atomic number", or number of protons and the number below is the "atomic weight", or the "Relative Atomic Mass".
Not every periodic table is the same though, generally the smaller number is the atomic number, although it should specify on the actual table you are using. The atomic number (on a non-ionic element) will also be the number of electrons, as generally and atom has the same amount of protons and neutrons. So like said prior it has no net charge.
Not every periodic table is the same though, generally the smaller number is the atomic number, although it should specify on the actual table you are using. The atomic number (on a non-ionic element) will also be the number of electrons, as generally and atom has the same amount of protons and neutrons. So like said prior it has no net charge.
To work out the number of neutrons an atom has, simply subtract the atomic number from the weight/relative atomic mass to get said neutron number.
I.e. for Carbon: 12 - 6 = 6. So 6 neutrons.
I hope you learned something or at least jogged your memory a bit, thanks for reading.
- Ben.

No comments:
Post a Comment